Ready-to-Use Pharmaceutical Packaging Market Trends 2035
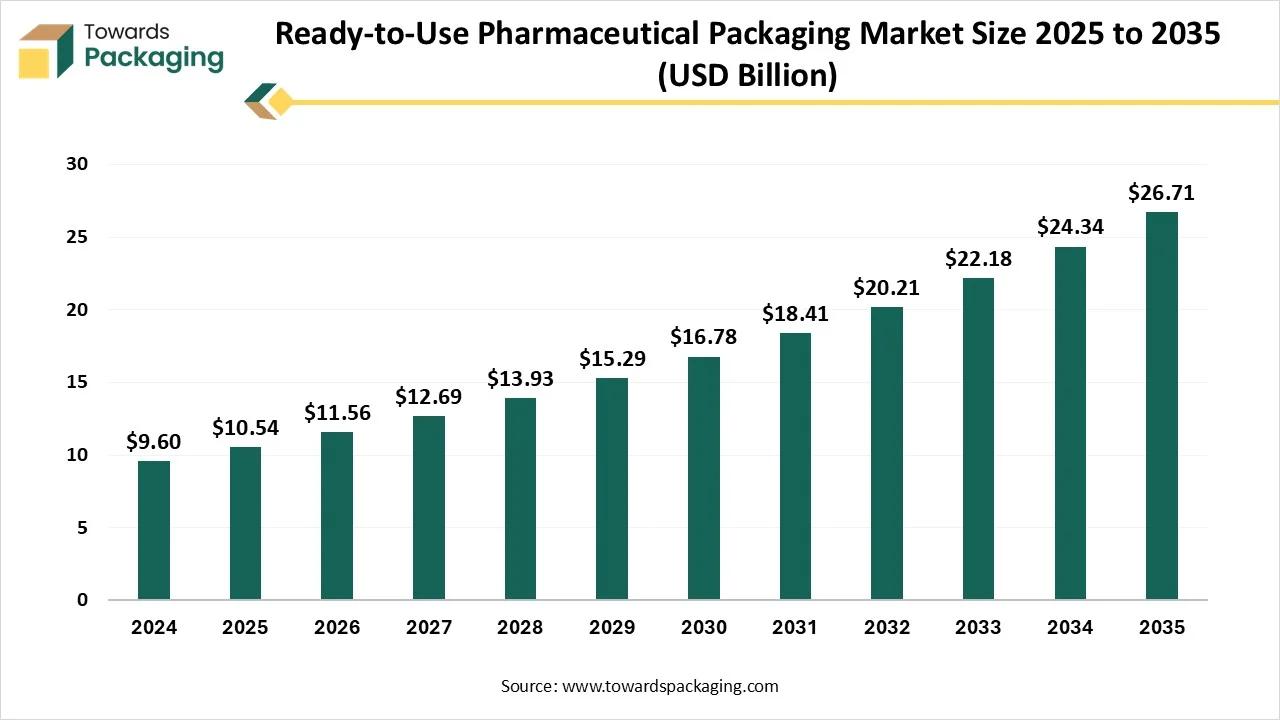
As reported by Towards Packaging experts, the global ready-to-use pharmaceutical packaging market is anticipated to rise from USD 11.56 billion in 2026 to approximately USD 26.71 billion by 2034, with a CAGR of 9.75% from 2025 to 2034.
Ottawa, Dec. 01, 2025 (GLOBE NEWSWIRE) -- The global ready-to-use pharmaceutical packaging market hit USD 10.54 billion in 2025, with current forecasts pointing to USD 26.71 billion by 2034, according to Towards Packaging, a sister firm of Precedence Research. The significance of the ready-to-use (RTU) pharmaceutical packaging market depends on its ability to raise efficiency, lower expenses, and improve safety for drug producers.
Request Research Report Built Around Your Goals: sales@towardspackaging.com
What is Meant by Ready-to-Use Pharmaceutical Packaging?
Ready-to-use (RTU) pharmaceutical packaging generally refers to pre-sterilized and pre-washed containers, like syringes, vials, and cartridges, which are ready for immediate filling with a drug product. This eliminates the demand for producers to perform in-house steps such as washing, sterilizing, and depyrogenating, which saves time, decreases operational complexity, and minimizes the risk of contamination. The main drivers for ready-to-use (RTU) pharmaceutical packaging are the rising focus on patient safety and product integrity, the need for operational efficiency and speed, and the demand to comply with strict regulatory requirements.
Other factors include the growth of complex drugs such as biologics, the growth of outsourced manufacturing, and the drive for personalized medicine. By removing the time-consuming and also expensive steps of washing along sterilizing containers, RTU packaging thus, streamlines the filling process, mainly for high-value drugs, biologics, and even personalized medicines.
What are the Latest Trends in the Ready-to-Use Pharmaceutical Packaging Market?
-
Emphasis on Recyclable and Biodegradable Materials
Consumers are more aware of environmental issues such as plastic pollution and are actively seeking goods from firms with strong sustainability commitments. Governments are thus introducing regulations, while pharmaceutical firms are taking corporate responsibility to heart by actively trying to decrease their environmental impact and even carbon footprint. Advances in biodegradable plastics, derived from renewable resources such as corn starch and sugarcane, enable materials that can offer the necessary protection and then break down naturally over time.
What Potentiates the Growth of the Ready-to-Use Pharmaceutical Packaging Industry?
-
Increasingly Stringent Regulatory Standards
Regulatory bodies such as the FDA and EMA inflict strict rules to protect patients. RTU packaging, which is usually pre-sterilized, ensures that the product remains sterile as well as safe from contamination via the supply chain, mainly for sensitive products such as injectables and biologics. Regulations demand packaging to protect drugs from environmental factors such as light, moisture, and damage during transit and storage. RTU packaging offers robust solutions that can be validated to meet such rigorous protection standards.
Get All the Details in Our Solutions - Access Report Sample: https://www.towardspackaging.com/download-sample/5876
Regional Analysis
Who is the Leader in the Ready-to-Use Pharmaceutical Packaging Market?
North America leads the market due to its strong pharmaceutical production base, high need from an aging population with chronic diseases, and advanced technology adoption, such as digital serialization and automation. Stringent regulations from agencies such as the FDA and USP also drive the demand for high-quality, sterilized, and even compliant packaging. The growth is thus fueled by significant investment in biologics and even personalized medicine, which need advanced, ready-to-use components to ensure sterility and also efficiency.
U.S. Ready-to-Use Pharmaceutical Packaging Market Trends
The U.S. market is rising, driven by the need for injectables and even personalized medicine, with sterile vials and syringes being considered the most popular container types. Key trends involve the adoption of RTU components to enhance operational efficiency and regulatory compliance, the increasing usage of advanced materials such as cyclic olefin polymers for improved product protection, and the integration of automation along with smart technologies for traceability and quality control.
Canada Market Trends
Canada's market is rising, driven by trends such as increasing need for sterile, high-quality packaging, along with advancements in biologics and personalized medicine, and a drive for sustainable and smart packaging solutions. Key trends include the growth of parenteral containers and even sterile syringes, the rising importance of specialized packaging for biologics, the acceptance of smart packaging technologies such as RFID, and a focus on eco-friendly materials to meet regulatory requirements, together with sustainability goals.
How is the Opportunistic Rise of Asia Pacific in the Ready-to-Use Pharmaceutical Packaging Industry?
Asia Pacific is driven by rising healthcare investment, growing drug demand, and even advancements in technology. Key factors involve government initiatives, the rising production of biologics and even specialty drugs, and a shift toward patient safety along with sterile solutions, which have boosted the need for sterile syringes, vials, and a few RTU containers. The development of complex biologics along with personalized medicines creates need for specialized, high-barrier, and even temperature-controlled packaging solutions.
China Ready-to-Use Pharmaceutical Packaging Market Trends
Key trends in China's market include a remarkable market growth boosted by the expanding pharmaceutical sector, a strong shift towards high-quality, sterile materials such as borosilicate glass, and rising adoption of smart packaging with serialization to combat counterfeiting along while meeting regulatory standards.
India Market Trends
The Indian market is rising rapidly, driven by the need for faster production, increased acceptance of biologics and personalized medicines, and even strict regulatory compliance. Key trends involve a shift to sterile, ready-to-use solutions to streamline production, a rise in smart packaging for anti-counterfeiting along with traceability, and a target on sustainability through eco-friendly materials.
More Insights of Towards Packaging:
- E-Commerce Packaging Market Size, Share, Trends, Segments, Regional Outlook (NA, EU, APAC, LA, MEA), and Competitive Analysis 2025-2034
- Pharmaceutical Temperature Controlled Packaging Solutions Market Size, Segments, Regional Insights (NA, EU, APAC, LA, MEA), and Competitive Landscape 2025-2034
- Protective Packaging Market Size, Segments, Regional Insights and Competitive Landscape Forecast to 2034
- AI in the Packaging Market Size, Segmentation, Regional Outlook, and Competitive Landscape Report 2025-2034
- Eco-friendly Packaging Market Size, Segments, Regions (NA, EU, APAC, LA, MEA), Companies & Competitive Landscape, Value Chain, Trade, Manufacturers & Suppliers
- Sachet Packaging Market Size, Segments, and Regional Insights: Comprehensive Industry Analysis (2024-2034)
- Metal Packaging Market Size, Share, Trends, Segments, and Regional Data (2025-2034)
- Mushroom Packaging Market Size, Segments, Regional Data, Competitive Landscape and Value Chain Analysis
- Liquid Packaging Market Size, Segmentation, Competitive Landscape, and Growth Opportunities (2025-2034)
- Water-Soluble Packaging Market Size, Share, Growth Forecast, Key Segments and Regional Insights (2025-2034)
- Agriculture Packaging Market Size, Key Segments by Material, End-Use, and Region and Competitive Landscape
- Food Packaging Technology and Equipment Market Size, Key Players, and Growth Prospects
- Feed Packaging Market Regional Insights, Segmentation, Materials, End Users and Key Players
- Digital Printing Packaging Market Size, Share, Segmentation, Trends, and Regional Performance (2025-2034)
-
Ready-To-Drink Packaging Market Size, Segments, Manufacturers, Value Chain and Competitive Analysis
Segment Outlook
Packaging Type Insights
Why did the Pre-Filled Syringes Segment Dominate the Ready-to-Use Pharmaceutical Packaging Market in 2024?
Due to a combination of patient safety, convenience, along clinical advantages. These syringes decrease the risk of contamination and even needle-stick injuries, provide precise pre-measured dosages for better patient compliance, along simplify administration, mainly for home use and chronic disease management.
Material Type Insights
Why did the Glass Packaging Segment Dominate the Ready-to-Use Pharmaceutical Packaging Market in 2024?
Glass provides superior barrier properties, protecting the contents from external influences such as gases and moisture. It is chemically inert along resistant to degradation, which prevents contamination and even preserves the drug's integrity over long periods. With a global drive for sustainable packaging, glass's recyclability makes it an attractive alternative for pharmaceutical companies looking to decrease their environmental impact.
Drug Type / Application Insights
Why did the Biologics & Vaccines Segment Dominate the Ready-to-Use Pharmaceutical Packaging Market in 2024?
These products are sensitive, high-value, and even require sterile, high-integrity packaging for safety and stability. The expansion of complex treatments such as biologics, biosimilars, and advanced therapies has created a remarkable need for RTU formats like sterile vials and prefilled syringes, which decrease labor, minimize contamination risks, and are even essential for self-administration. Strict guidelines from regulatory agencies such as the FDA and EMA mandate high standards for sterility along with product integrity, which prompts pharmaceutical companies to accept RTU packaging to meet compliance requirements.
The oncology drugs segment is the fastest-growing in the market during the forecast period.
Due to the rising global cancer rates and the development of complex, high-value oncology drugs that demand specialized packaging. These factors drive the need for advanced, sterile RTU packaging solutions such as prefilled syringes and vials, which guarantee drug stability, safety, and even faster market entry. Many modern cancer treatments, includes biologics and cell and gene therapies, are complex and demand specialized primary packaging, like prefilled syringes and vials, to maintain sterility and stability.
End User Insights
Why did the Hospitals & Clinics Segment Dominate the Ready-to-Use Pharmaceutical Packaging Market in 2024?
This is because hospitals and clinics are places where the majority of pharmaceuticals are administered, and even they depend on RTU packaging for reasons such as patient safety, efficiency, and also regulatory compliance. By eliminating the demand for in-house sterilization and preparation, RTU packaging permits healthcare professionals to administer medication more quickly, which is vital in emergencies.
The retail pharmacies / specialty pharmacies segment is the fastest-growing in the market during the forecast period.
Due to the rise in need for specialty drugs, raised chronic diseases, and even the convenience and accessibility of pharmacies for patients. As more complex and high-value drugs are developed, along with there is a corresponding increase in the demand for RTU packaging that ensures sterility and also integrity for both retail together with specialty pharmacy distribution. The impact on patient safety and strict regulatory guidelines from agencies the FDA, necessitate the usage of pre-sterilized packaging to guarantee product integrity and sterility from the manufacturer to the patient.
Elevate your packaging strategy with Towards Packaging. Enhance efficiency and achieve superior results - schedule a call today: https://www.towardspackaging.com/schedule-meeting
Recent Breakthroughs in the Global Ready-to-Use Pharmaceutical Packaging Industry
- In April 2025, Syntegon started the MLD Advanced, a new filling machine engineered for RTU nested syringes, to meet pharmaceutical producers' growing need for high-output and precise filling. The MLD Advanced is designed to meet pharmaceutical manufacturers’ requirements of high output with 100% in-process control (IPC).
Top Companies in the Ready-to-Use Pharmaceutical Packaging Market & Their Offerings:
- Catalent, Inc.: Offers integrated CDMO services, including aseptic filling and packaging of drug products into RTU vials, syringes, and cartridges.
- Becton Dickinson (BD): Provides a wide range of RTU pre-fillable syringes, vials, and innovative on-body wearable injectors.
- Ypsomed AG: Specializes in developing and manufacturing self-injection systems like autoinjectors, pen injectors, and patch injectors designed for use with pre-filled, RTU primary containers.
- Terumo Corporation: Delivers superior quality ready-to-fill (RTF) polymer syringes and primary packaging solutions that are sterile and ready for filling with demanding drug formulations.
- Stevanato Group: Supplies high-quality RTU glass primary packaging (vials, cartridges, syringes) and integrated container closure systems.
- HCT Group: Primarily a packaging solutions provider for the cosmetics and beauty industries, not a key player in pharmaceutical packaging.
- AptarGroup: Provides innovative dispensing and active packaging solutions, including specialized stoppers and components for pharmaceutical drug delivery systems.
- Amcor Limited: Offers a wide array of flexible and rigid packaging solutions for pharmaceutical and medical products, focusing on both primary and secondary packaging.
- Daikyo Seiko, Ltd.: Manufactures RTU components such as plungers, stoppers, and caps for various primary containers like pre-fillable syringes.
- SHL Group: Focuses on the design and manufacture of advanced autoinjectors and other drug delivery systems, which are later assembled with the drug.
- Unilife Corporation: The company is no longer in business, having gone bankrupt in 2017 with assets acquired by other entities.
- Corning Inc.: Offers high-quality glass tubing and vials for pharmaceutical applications, including Gx RTU vials designed for enhanced drug containment and quality.
Segments Covered in the Report
By Packaging Type
- Pre-Filled Syringes
- Vials (Single-use / Multi-dose)
- Cartridges & Ampoules
- Pre-Filled Bags (e.g., infusion bags)
- Others (Ready-to-Use kits, combination devices)
By Material Type
- Glass Packaging
- Plastic / Polymer Packaging
- Hybrid / Multi-layer Materials
- Others (biodegradable / sustainable polymers)
By Drug Type / Application
- Biologics & Vaccines
- Small Molecule Injectable Drugs
- Oncology Drugs
- Others (antibiotics, hormones, anesthetics)
By End User
- Hospitals & Clinics
- Contract Manufacturing Organizations (CMOs) / Pharmaceutical Manufacturers
- Retail Pharmacies / Specialty Pharmacies
- Homecare / Patient-administered Settings
By Region
-
North America:
- U.S.
- Canada
- Mexico
- Rest of North America
-
South America:
- Brazil
- Argentina
- Rest of South America
-
Europe:
-
Western Europe
- Germany
- Italy
- France
- Netherlands
- Spain
- Portugal
- Belgium
- Ireland
- UK
- Iceland
- Switzerland
- Poland
- Rest of Western Europe
-
Western Europe
-
Eastern Europe
- Austria
- Russia & Belarus
- Türkiye
- Albania
- Rest of Eastern Europe
-
Asia Pacific:
- China
- Taiwan
- India
- Japan
- Australia and New Zealand,
- ASEAN Countries (Singapore, Malaysia)
- South Korea
- Rest of APAC
-
MEA:
- GCC Countries
- Saudi Arabia
- United Arab Emirates (UAE)
- Qatar
- Kuwait
- Oman
- Bahrain
- South Africa
- Egypt
- Rest of MEA
Invest in Our Premium Strategic Solution: https://www.towardspackaging.com/checkout/5876
Request Research Report Built Around Your Goals: sales@towardspackaging.com
About Us
Towards Packaging is a global consulting and market intelligence firm specializing in strategic research across key packaging segments including sustainable, flexible, smart, biodegradable, and recycled packaging. We empower businesses with actionable insights, trend analysis, and data-driven strategies. Our experienced consultants use advanced research methodologies to help companies of all sizes navigate market shifts, identify growth opportunities, and stay competitive in the global packaging industry.
Stay Connected with Towards Packaging:
- Find us on Social Platforms: LinkedIn | Twitter | Instagram | Threads
- Subscribe to Our Newsletter: Towards Sustainable Packaging
- Visit Towards Packaging for In-depth Market Insights: Towards Packaging
- Read Our Printed Chronicle: Packaging Web Wire
-
Get ahead of the trends – follow us for exclusive insights and industry updates:
Pinterest | Medium | Tumblr | Hashnode | Bloglovin | LinkedIn – Packaging Web Wire | Globbook | Substack | Bluesky | - Contact: APAC: +91 9356 9282 04 | Europe: +44 778 256 0738 | North America: +1 8044 4193 44
Our Trusted Data Partners
Precedence Research | Statifacts | Towards Healthcare | Towards Auto | Towards Food and Beverages | Towards Chemical and Materials | Towards Consumer Goods | Towards Dental | Towards EV Solutions | Nova One Advisor | Healthcare Webwire | Packaging Webwire | Automotive Webwire | Nutraceuticals Func Foods | Onco Quant | Sustainability Quant | Specialty Chemicals Analytics
Towards Packaging Releases Its Latest Insight - Check It Out:
- Vacuum Packaging Market Size, Competitive Landscape, Key Manufacturers, and Suppliers Data 2034
- Thermoform Packaging Market Size, Share, Trends, and Competitive Analysis 2025-2034
- Blister Packaging Market Growth, Key Segments, and Regional Dynamics with Manufacturers and Suppliers Data
- Security Printing Services Market Size, Segments, Companies, Value Chain & Trade Analysis 2025-2034
- Child Resistant Packaging Market Size, Share, Trends, Segments, and Regional Insights (NA, EU, APAC, LA, MEA)
- Pharmaceutical Packaging Equipment Market Size, Segments, Companies, Competitive Analysis, Value Chain & Trade Analysis 2025-2034
- Asia Pacific Food Packaging Market Growth, Key Segments, and Regional Dynamics with Manufacturers and Suppliers Data
- North America Pharmaceutical Packaging Market Growth, Key Segments, and Regional Dynamics with Manufacturers and Suppliers Data
- Stick Packaging Market Size, Segments, Companies, Competitive Analysis, Value Chain & Trade Analysis 2025-2034
- Dairy Product Packaging Market Size, Segments, Share and Companies (2025-34)
- Baby Food Packaging Market Size, Share, Trends, Segments, and Regional Insights (NA, EU, APAC, LA, MEA)
- North America Automotive Thermoformed Plastic Parts Packaging Market Growth, Key Segments and Regional Dynamics
- Europe Glass Prefilled Syringes And Glass Vials Packaging Equipment Market Growth, Key Segments, and Suppliers Data
- Reusable Packaging Market Size, Segments, Regional Data (NA/EU/APAC/LA/MEA), Companies, Competitive Analysis, Value Chain & Trade Analysis 2025-2034
-
Recyclable Packaging Market Size, Segmentation, and Competitive Landscape Analysis

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
